Hydrogen abstraction by photoexcited benzophenone: consequences for DNA photosensitization†
Abstract
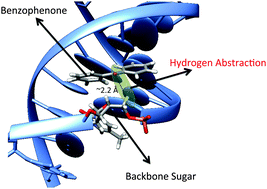
We report a computational investigation of the hydrogen abstraction (H-abstraction) induced by triplet benzophenone (3BP) on thymine nucleobase and backbone sugar. The chemical process is studied using both high level multiconfigurational perturbation and density functional theory. Both methods show good agreement in predicting small kinetic barriers. Furthermore the behavior of benzophenone in DNA is simulated using molecular dynamics and hybrid quantum mechanics/molecular mechanics methods. The accessibility of benzophenone to the labile hydrogens within B-DNA is demonstrated, as well as the driving force for this reaction. We evidence a strong dependence of the H-abstraction with the non-covalent BP–DNA interaction mode, and a reaction that is less favorable when embedded in DNA than for the isolated system.

 Please wait while we load your content...
Please wait while we load your content...