Facile preparation of β-/γ-MgH2 nanocomposites under mild conditions and pathways to rapid dehydrogenation†
Abstract
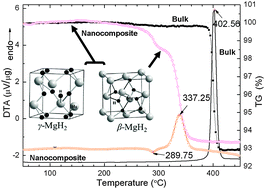
A magnesium hydride composite with enhanced hydrogen desorption kinetics can be synthesized via a simple wet chemical route by ball milling MgH2 with LiCl as an additive at room temperature followed by tetrahydrofuran (THF) treatment under an Ar atmosphere. The as-synthesized composite comprises ca. 18 mass% orthorhombic γ-MgH2 and 80 mass% tetragonal β-MgH2 as submicron-sized particles. The β-/γ-MgH2 nanocomposite exhibits a dehydrogenation capacity of 6.6 wt% and starts to release hydrogen at ∼260 °C; ca. 140 °C lower than that of commercial MgH2. The apparent activation energy for dehydrogenation is 115 ± 3 kJ mol−1, which is ca. 46% lower than that of commercial MgH2. Analysis suggests that the meta-stable γ-MgH2 component either directly dehydrogenates exothermically or first transforms into stable β-MgH2 very close to the dehydrogenation onset. The improved hydrogen release performance can be attributed both to the existence of the MgH2 nanostructure and to the presence of γ-MgH2.

 Please wait while we load your content...
Please wait while we load your content...