Antiferromagnetic FeMn alloys electrodeposited from chloride-based electrolytes
Abstract
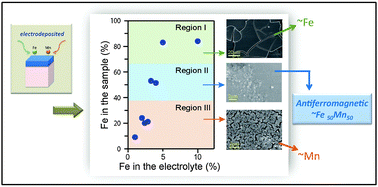
The capability of synthesizing Fe-based antiferromagnetic metal alloys would fuel the use of electrodeposition in the design of new magnetic devices such as high-aspect-ratio spin valves or new nanostructured hard magnetic composites. Here we report the synthesis of high quality antiferromagnetic FeMn alloys electrodeposited from chloride-based electrolytes. We have found that in order to grow homogeneous FeMn films it is necessary to incorporate a large concentration of NH4Cl as an additive in the electrolyte. The study of the structure and magnetic properties shows that films with composition close to Fe50Mn50 are homogeneous antiferromagnetic alloys. We have established a parameter window for the synthesis of FeMn alloys that show antiferromagnetism at room temperature.

 Please wait while we load your content...
Please wait while we load your content...