Structure–property relationships in two-dimensionally extended benzoporphyrin molecules probed using single-molecule fluorescence spectroscopy†
Abstract
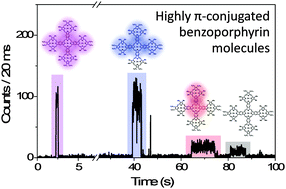
The photophysical properties of a series of highly π-conjugated benzoporphyrin molecules (BPNs) with different shapes were investigated in the condensed phase using single-molecule fluorescence spectroscopy. The fluorescence properties of single BPNs were found to be affected by the number of porphyrin units and their molecular shapes. Notably, the single-molecule fluorescence dynamics of the BPNs revealed an increase in the fluorescence lifetimes and blue shifts of the fluorescence spectra indicative of decreasing π-conjugation pathways in the molecules. The distributions of the spectroscopic parameters and the photostability for the molecules also suggest conformational complexities and heterogeneities. Specifically, as the number of constituent porphyrin units increased, the one-step photobleaching behavior ratio and photostability decreased, and the spectroscopic parameter distributions broadened. The structural properties of the BPNs were also directly determined using defocused wide-field imaging and linear dichroism analyses. In particular, molecules with the same number of constituent porphyrins but different molecular shapes exhibited distinct photophysical properties. In summary, these observations provide guidance for the design of molecular systems that can enhance the performance of molecular electronic devices.

 Please wait while we load your content...
Please wait while we load your content...