Understanding adsorption of CO2, N2, CH4 and their mixtures in functionalized carbon nanopipe arrays†
Abstract
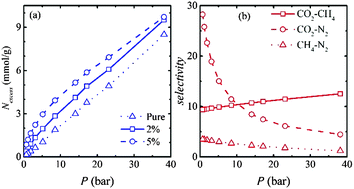
The selective adsorption behaviours of carbon dioxide, methane and nitrogen on bundles of functionalized CMK-5 are investigated at 303 K using grand-canonical Monte Carlo simulations. Functional groups (–OH, –COOH) cause a significant enhancement in CO2 uptake (up to 19.5% at a pressure of 38.13 bar for –COOH). On the other hand, the adsorption amount of methane decreases with respect to bare CMK-5 by ∼13% (at 38.13 bar) upon functionalization. Furthermore, functionalized CMK-5 with different pore sizes (4 nm, 6 nm, 8 nm) and inter-tube distances (d = 0 to 1.5 nm) are used to investigate the adsorption behaviour of flue gases. While the pore diameter is seen to reduce the isosteric heat of adsorption, the inter-tube distance of 0.25 nm shows the highest uptake of CO2 at p ≤ 18 bar, followed by 0.5 nm for the pressure range of 18 < p ≤ 30 bar, whereas for p > 30 bar, d = 1.0 nm shows the maximum uptake. For methane and nitrogen, the maximum adsorption is obtained at d = 0.25 nm in the studied pressure range. The selective adsorption of CO2 in binary mixtures is investigated using ideal adsorption solution theory. CO2–N2 selectivity is found to increase significantly by surface functionalization of CMK-5 compared to pure CMK-5. The maximum selectivity of CO2–CH4 using –COOH functionalized CMK-5 is found to be ∼10 for an equimolar CO2–CH4 mixture at a pressure of 38.13 bar.

 Please wait while we load your content...
Please wait while we load your content...