A two-oxide nanodiode system made of double-layered p-type Ag2O@n-type TiO2 for rapid reduction of 4-nitrophenol
Abstract
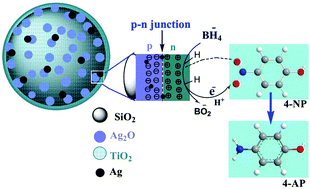
The n-type TiO2 semiconductor nanoparticles were coated on the p-type Ag2O nanoparticles deposited on SiO2 spherical particles through a simple sol–gel method for catalytic reduction of 4-nitrophenol. The as-prepared spherical composite abbreviated as SiO2/Ag2O@TiO2 was characterized by different techniques and tested as a catalyst towards 4-nitrophenol (4-NP) reduction into 4-aminophenol (4-AP) with NaBH4 as a reducing agent at room temperature. This work combines an interesting design with the n-type TiO2 rich in electrons outward and the p-type Ag2O rich in electronic holes inward to form the p/n junction for the purpose of efficiently separating the charge carrier to have a longer lifetime of outward electrons for catalytic reduction reactions. The SiO2/Ag2O@TiO2 composite catalyst showed the best performance in the reduction of 4-NP to 4-AP within 30 seconds. Our results reveal that the p–n junction combined composite sphere was superior and efficient in reduction of 4-nitrophenol without using the light source. The conversion mechanism is proposed here. Overall, the SiO2/Ag2O@TiO2 composite can be used as a cost-effective reduction catalyst for converting the toxic 4-NP into useful 4-AP, an industrial organic intermediate compound.
- This article is part of the themed collection: 2016 most accessed PCCP articles

 Please wait while we load your content...
Please wait while we load your content...