Impact of chirality on the photoinduced charge transfer in linked systems containing naproxen enantiomers†
Abstract
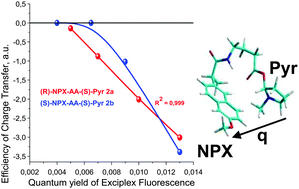
The model reaction of photoinduced donor–acceptor interaction in linked systems (dyads) has been used to study the comparative reactivity of a well-known anti-inflammatory drug, (S)-naproxen (NPX) and its (R)-isomer. (R)- or (S)-NPX in these dyads is linked to (S)-N-methylpyrrolidine (Pyr) using a linear or cyclic amino acid bridge (AA or CyAA), to give (R)-/(S)-NPX–AA–(S)-Pyr flexible and (R)-/(S)-NPX–CyAA–(S)-Pyr rigid dyads. The donor–acceptor interaction is reminiscent of the binding (partial charge transfer, CT) and electron transfer (ET) processes involved in the extensively studied inhibition of the cyclooxygenase enzymes (COXs) by the NPX enantiomers. Besides that, both optical isomers undergo oxidative metabolism by enzymes from the P450 family, which also includes ET. The scheme proposed for the excitation quenching of the (R)- and (S)-NPX excited state in these dyads is based on the joint analysis of the chemically induced dynamic nuclear polarization (CIDNP) and fluorescence data. The 1H CIDNP effects in this system appear in the back electron transfer in the biradical–zwitterion (BZ), which is formed via dyad photoirradiation. The rate constants of individual steps in the proposed scheme and the fluorescence quantum yields of the local excited (LE) states and exciplexes show stereoselectivity. It depends on the bridge's length, structure and solvent polarity. The CIDNP effects (experimental and calculated) also demonstrate stereodifferentiation. The exciplex quantum yields and the rates of formation are larger for the dyads containing (R)-NPX, which let us suggest a higher contribution from the CT processes with the (R)-optical isomer.

 Please wait while we load your content...
Please wait while we load your content...