Mechanistic investigation of trimethylamine-N-oxide reduction catalysed by biomimetic molybdenum enzyme models†
Abstract
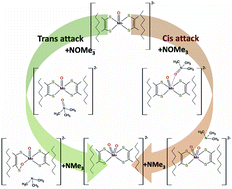
In this paper, we report a theoretical investigation of the reduction reaction mechanism of Me3NO using molybdenum containing systems that are functional and structural analogues of trimethylamine N-oxide reductase mononuclear molybdenum enzyme. The reactivity of the monooxomolybdenum(IV) benzenedithiolato complex and its derivatives to carbamoyl (t-BuNHCO) and acylamino (t-BuCONH) substituents on the benzene rings in both cis and trans arrangements was explored. The calculated energy profiles describing the steps of two mechanisms of attack considered viable (named cis- and trans-attack) by the Me3NO substrate at cis and trans positions with respect to the oxo ligand show that the attack on cis is energetically more favourable than the attack on trans. Along the pathway for the cis-attack the first step of the reaction, that is rate-determining for all the studied compounds, is the approach of the substrate to the Mo centre in cis to the oxo ligand that causes a distortion of the initial square-pyramidal geometry of the complex. The reaction steps involved in the trans position attack were also explored. Calculations confirm that, as previously suggested, the introduction of ligands able to form intramolecular NH⋯S hydrogen bonds accelerates the reduction of the Me3NO substrate and contributes to the tuning of the reactivity of molybdoenzyme models.

 Please wait while we load your content...
Please wait while we load your content...