Production of renewable fuels by the photohydrogenation of CO2: effect of the Cu species loaded onto TiO2 photocatalysts
Abstract
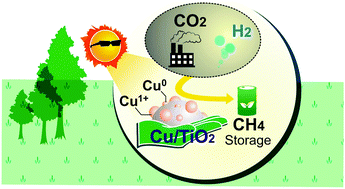
The efficient gas phase photocatalytic hydrogenation of CO2 into a desirable renewable fuel was achieved using a Cu-loaded TiO2 photocatalyst system. Enhancing the amount of Ti3+ relative to Ti4+ in a Cu-loaded TiO2 photocatalyst provided an excellent opportunity to promote the photohydrogenation of CO2. The coexistence of Cu and Cu+ species during the photoreaction was shown to efficiently enhance the photocatalytic activity by prolonging the lifetime of the electrons. To achieve the best photoactivity, the Cu species must be maintained at an appropriately low concentration (≤1 wt%). The highest CH4 yield obtained was 28.72 μmol g−1. This approach opens a feasible route not only to store hydrogen by converting it into a desirable renewable fuel, but also to reduce the amount of the greenhouse gas CO2 in the atmosphere.

 Please wait while we load your content...
Please wait while we load your content...