Electron delocalization and electron density of small polycyclic aromatic hydrocarbons in singlet excited states†
Abstract
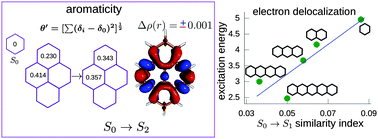
The four lowest singlet electronic states of benzene, the acenes from naphthalene to pentacene, phenanthrene and pyrene were studied by means of theoretical methods. Their vertical excitation energies from the ground electronic states were computed at the CASPT2 approximation. As an attempt to explain the trends observed in the excitation energies, several descriptors based on the electron density were used and the similarity of these molecules with their ground state counterparts was analyzed. It was found that the changes of the topological properties at the C–C bond critical points do not explain the decreasing trends for the excitation energies with the increase of the number of rings, in part because the small changes that take place in the electron density occur above and below the molecular plane. A similarity index based on electron delocalization between quantum topological atoms was defined to compare a molecule in two different electronic states. It was found that, mainly for the acenes, this index goes in line with the excitation energies to the first excited state. Implications of the changes in electron delocalization on the aromatic character of these molecules are also discussed. In general, local aromaticity decreases upon excitation.
- This article is part of the themed collection: Electron delocalization and aromaticity: 150 years of the Kekulé benzene structure

 Please wait while we load your content...
Please wait while we load your content...