Charge retention of soft-landed phosphotungstate Keggin anions on self-assembled monolayers†
Abstract
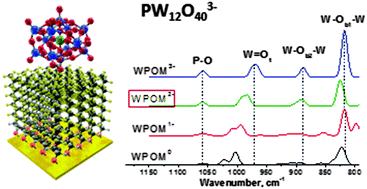
Soft landing of mass-selected ions onto surfaces often results in partial loss of charge that may affect the structure and reactivity of deposited species. In this study, Keggin phosphotungstate anions in two selected charge states, PW12O403− (WPOM3−) and PW12O402− (WPOM2−), were soft-landed onto different self-assembled monolayer (SAM) surfaces and examined using in situ infrared reflection absorption spectroscopy (IRRAS) and density functional theory (DFT) calculations. Partial retention of the 3− charge was observed when WPOM3− was soft-landed onto the fluorinated SAM (FSAM), while the charge state distribution was dominated by the 2− charge after both WPOM3− and WPOM2− were deposited onto a hydrophilic alkylthiol SAM terminated with cationic NH3+ functional groups (NH3+SAM). We found that during the course of the soft landing of WPOM3−, the relative abundance of WPOM3− on FSAM decreased while that of WPOM2− increased. We propose that the higher stability of immobilized WPOM2− in comparison with WPOM3− makes it the preferred charge state of WPOM on both the FSAM and NH3+SAM. We also observe weaker binding of WPOM anions to SAMs in comparison with phosphomolybdate ions (MoPOM) reported previously (J. Phys. Chem. C, 2014, 118, 27611–27622). The weaker binding of WPOM to SAMs is attributed to the lower reactivity of WPOM reported in the literature. This study demonstrates that both the charge retention and the reactivity of deposited anionic POM clusters on surfaces are determined by the type of addenda metal atoms in the cluster.

 Please wait while we load your content...
Please wait while we load your content...