Methanol synthesis via CO2 hydrogenation over a Au/ZnO catalyst: an isotope labelling study on the role of CO in the reaction process†
Abstract
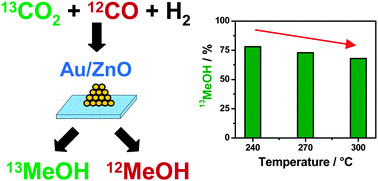
Methanol synthesis for chemical energy storage, via hydrogenation of CO2 with H2 produced by renewable energies, is usually accompanied by the undesired formation of CO via the reverse water–gas shift reaction. Aiming at a better mechanistic understanding of methanol formation from CO2/H2 on highly selective supported Au/ZnO catalysts we have investigated the role of CO in the reaction process using isotope labelling experiments. Using 13C-labelled CO2, we found for reaction at 5 bar and 240 °C that (i) the methanol formation rate is significantly higher in CO2-containing gas mixtures than in a CO2-free mixture and (ii) in mixtures containing both CO2 and CO methanol formation from CO increases with the CO content up to 1% CO, and then remains at 20% of the total methanol formation up to a CO2/CO ratio of 1/1, making CO2 the preferred carbon source in these mixtures. A shift in the preferred carbon source for MeOH from CO2 towards CO is observed with increasing reaction temperatures between 240 °C and 300 °C. At even higher temperatures CO is expected to become the dominant carbon source. The consequences of these findings for the application of Au/ZnO catalysts for chemical storage of renewable energies are discussed.
- This article is part of the themed collection: Bunsentagung 2016: Basic Mechanisms in Energy Conversion

 Please wait while we load your content...
Please wait while we load your content...