Two photon absorption properties of four coordinated transition metal complexes of tetraarylazadipyrromethene compounds†
Abstract
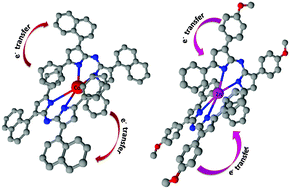
New tetraarylazadipyrromethene metal complexes with four coordinate metals (cobalt(II), nickel(II), copper(II) and zinc(II)) and with three moieties (4-methylphenyl,4-methoxyphenyl and 1-naphthyl) were designed and synthesized targeting applications utilizing two photon absorption. The effects of metals with filled or unfilled d orbitals and substituents with various electron donor properties on the charge transfer mechanism and two photon absorption properties of tetraarylazadipyrromethene compounds were investigated by ultrafast pump–probe spectroscopy and open aperture Z-scan experiments as well as density functional theory (DFT) calculations. Ultrafast transient absorption spectra provide evidence of an efficient photoinduced intramolecular charge transfer between the ligand and metals which is independent of filled or unfilled d orbitals of metals. Although zinc has filled d orbitals, its complexes possess an absorption maximum including a shoulder which is attributed to partial ligand to metal L(π) → M(d*) charge transfer character (LMCT). Due to the charge transfer mechanism, metal complexes of tetraarylazadipyrromethene compounds exhibited two photon absorption properties in the femtosecond regime at 800 nm wavelength. The greatest two photon absorption cross section value was measured as 2690 GM for Zn(L2)2 and 2374 GM for Co(L3)2 complexes.

 Please wait while we load your content...
Please wait while we load your content...