Shape selective properties of the Al-fumarate metal–organic framework in the adsorption and separation of n-alkanes, iso-alkanes, cyclo-alkanes and aromatic hydrocarbons†
Abstract
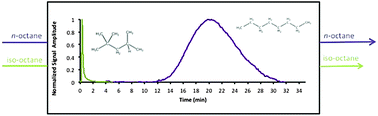
The primary goal of this work is to study the adsorption of a wide range of hydrocarbon adsorbates in the Al-fumarate metal–organic framework in order to identify and explore trends in adsorption behaviour that can be related to the sorbate's molecular properties and as well as the properties of this MOF. The pulse chromatographic technique was used to study the adsorption properties of C5–C8 linear, branched, cyclic and aromatic hydrocarbons in vapour phase at low coverage and at high temperatures (150–250 °C). Chromatograms of alkanes having the same number of carbon atoms (C5–C8) clearly show that the linear alkane is retained the longest over its branched and cyclic isomers. Moreover, xylene isomers are also clearly separated by Al-fumarate, with retention times increasing in the order: ortho-xylene < meta-xylene < para-xylene. Differences in adsorption enthalpy of more than 10 kJ mol−1 between linear alkanes and their di/tri-branched or cyclo-alkane isomers were observed, clearly showing that steric effects imposed by the pore structure of the adsorbent cause the difference in adsorption between linear alkanes and their isomers. In conclusion, Al-fumarate behaves as a shape selective material with respect to structural isomers of linear alkanes, with properties resembling those of medium pore size zeolites.

 Please wait while we load your content...
Please wait while we load your content...