Proline cis–trans isomerization and its implications for the dimerization of analogues of cyclopeptide stylostatin 1: a combined computational and experimental study†
Abstract
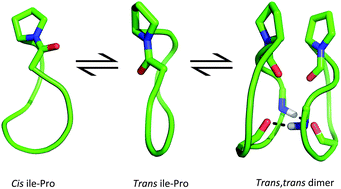
Cis and trans proline conformers are often associated with dramatic changes in the biological function of peptides. A slow equilibrium between cis and trans Ile–Pro amide bond conformers occurs in constrained derivatives of the native marine cyclic heptapeptide stylostatin 1 (cyclo-(NSLAIPF)), a potential anticancer agent. In this work, four cyclopeptides, cyclo-(NSTAIPF), cyclo-(KSTAIPF), cyclo-(RSTAIPF) and cyclo-(DSTAIPF), which are structurally related to stylostatin 1, are experimentally and computationally examined in order to assess the effect of residue mutations on the cis–trans conformational ratio and the apparent capacity to form dimeric aggregates. Primarily, cyclo-(KSTAIPF) and cyclo-(RSTAIPF) showed specific trends in circular dichroism, MALDI-TOF and HPLC purification experiments, which suggests the occurrence of peptide dimerization. Meanwhile, the NMR spectrum of cyclo-(KSTAIPF) indicates that this cyclopeptide exists in the two slow-exchange families of conformations mentioned above. Molecular dynamics simulations combined with quantum mechanical calculations have shed light on the factors governing the cis/trans conformational ratio. In particular, we have found that residue mutations affect the internal hydrogen bond pattern which ultimately tunes the cis/trans conformational ratio and that only trans conformers are capable of aggregating due to the shape complementarity of the two subunits.

 Please wait while we load your content...
Please wait while we load your content...