Electrochemo-dynamical characterization of polypyrrole actuators coated on gold electrodes†
Abstract
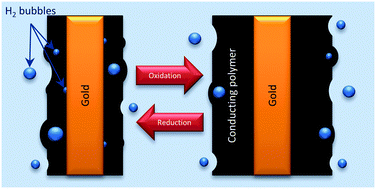
Polypyrrole coated gold wires were subjected to consecutive square current waves in LiClO4 aqueous solutions using the same constant anodic and cathodic charge. Parallel in situ diameter variations were followed using a laser scan micrometer. The procedure was repeated by changing one experimental variable every time: applied current, electrolyte concentration or working temperature to perform electrochemodynamical characterization of the system. On average, the diameter follows a linear variation of the consumed charge, as expected for any faradaic system, although a high dispersion was attained in the data. Such deviations were attributed to the presence of irreversible hydrogen evolution at the gold/polypyrrole interface at cathodic potentials more than 0.0 V vs. Ag/AgCl, detected and quantified from separated coulovoltammetric responses. Despite this parallel hydrogen evolution the consumed energy during reactions is a robust sensor of the working conditions. In conclusion a gold support, the metal most used for technological applications of conducting polymers, should be avoided when a device is driven by current flow in the presence of aqueous solutions, water contamination or moisture: a fraction of the charge will be consumed by hydrogen generation with possible degradation of the device.


 Please wait while we load your content...
Please wait while we load your content...