Synthesis and spectral measurements of sulphonated graphene: some anomalous observations†
Abstract
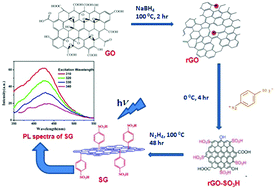
The present report demonstrates how a sulphonation process, a key route for synthesizing water soluble graphene, can influence the optical behavior of precursor graphene oxide, intermediate reaction products and sulphonated graphene. We observed that there is constant emission maximum at 500 nm for graphene oxide in the excitation range of 320–450 nm. During sulphonation, sulphonated reduced graphene oxide (rGO-SO3H) is initially formed which has an emission at 358 nm. However, the reduction of oxygen containing groups in rGO-SO3H with hydrazine hydrate leading to the formation of SG caused a shift in the emission to 430 nm, which has been attributed to the extended delocalization of π-electrons involving the phenyl sulphonate group. In the present investigation, we have identified many existing anomalies in the important spectral features of these materials, such as violation of Kasha's rule on fluorescence and pH dependence emission. Furthermore, it has also been shown that proper care is necessary to be taken in monitoring the fluorescence of sulphonated graphene in view of possible interference from the components produced during sulphonation.

 Please wait while we load your content...
Please wait while we load your content...