Synthesis, structure and the dehydrogenation mechanism of calcium amidoborane hydrazinates†
Abstract
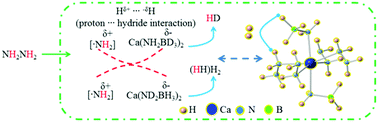
The calcium amidoborane hydrazinates, Ca(NH2BH3)2·nN2H4, were firstly synthesized by reacting different molar ratios of Ca(NH2BH3)2 and N2H4. In particular, Ca(NH2BH3)2 and N2H4 with a molar ratio of 1 : 2 crystallizes into the orthorhombic symmetry P212121 space group with the lattice parameters of a = 6.6239(4) Å, b = 13.7932(6) Å, c = 4.7909(2) Å. The dehydrogenations of calcium amidoborane hydrazinates are two-step reactions, exhibiting superior dehydrogenation properties compared with those of pristine Ca(NH2BH3)2. For Ca(NH2BH3)2–1/2N2H4, approximately 4.6 equiv. hydrogen (or 7.9 wt% hydrogen) can be released at 150 °C. Kinetic analysis shows that the activation energies for the two steps of hydrogen desorption from Ca(NH2BH3)2·2N2H4 are much lower than those of pristine Ca(NH2BH3)2, suggesting an improvement in the dehydrogenation kinetics of Ca(NH2BH3)2 after coordinating with N2H4. Isotopic labeling results show that the driving force for the dehydrogenation of calcium amidoborane hydrazinates is the combination mechanism of protonic hydrogen and hydridic hydrogen (Hδ+ and Hδ−). In addition, initial H2 release from calcium amidoborane hydrazinates originates from the interaction of [–BH3] and N2H4, rather than [–BH3] and [–NH2] (in [–NH2BH3]).

 Please wait while we load your content...
Please wait while we load your content...