Bombardment induced ion transport – part IV: ionic conductivity of ultra-thin polyelectrolyte multilayer films
Abstract
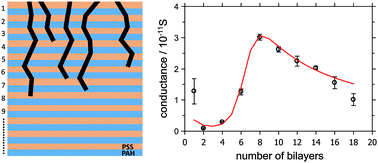
The dependence of the ionic conductance of ultra-thin polyelectrolyte multilayer (PEM) films on the temperature and the number of bilayers has been investigated by the recently developed low energy bombardment induced ion transport (BIIT) method. To this end multilayers of alternating poly(sodium 4-styrene sulfonate) (PSS) and poly(allylamine hydrochloride) (PAH) layers were deposited on a metal electrode and subsequently bombarded by a low energy potassium ion beam. Ions are transported through the film according to the laws of electro-diffusion towards a grounded backside electrode. They are neutralized at the interface between the polymer film and the metal electrode. The detected neutralization current scales linearly with the acceleration potential of the ion beam indicating Ohmic behavior for the (PAH/PSS)x multilayer, where x denotes the number of bilayers. The conductance exhibits a non-monotonic dependence on the number of bilayers, x. For 2 ≤ x ≤ 8 the conductance increases non-linearly with the number of bilayers. For x ≥ 8 the conductance decreases with increasing number of bilayers. The variation of the conductance is rationalized by a model accounting for the structure dependence of the conductivity. The thinnest sample for which the conductance has been measured is the single bilayer reflecting properties dominated by the interface. The activation energy for the ion transport is 0.49 eV.

 Please wait while we load your content...
Please wait while we load your content...