Hydrogen bond mediated stabilization of the salt bridge structure for the glycine dimer anion†
Abstract
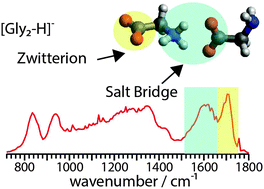
The formation of a salt bridge in deprotonated glycine dimer anions in a solvent-free environment is investigated using both infrared multiple photon dissociation spectroscopy between 600 and 1800 cm−1 and theory. The zwitterionic and nonzwitterionic forms of glycine in this complex are computed to be nearly iso-energetic, yet predominantly the zwitterionic form is observed experimentally. The zwitterion stability is attributed to both the Coulombic attraction and the high stabilization from intramolecular hydrogen bonding that drives the energetic cost of proton transfer in a solvent free environment. These results show that there is a fine balance between the stabilities of these two forms of the anion. Elucidating the role of intrinsic factors, such as hydrogen bonding, can lead to a better understanding of the stabilities of salt bridges in the interiors of large proteins or at protein interfaces.

 Please wait while we load your content...
Please wait while we load your content...