Application-oriented computational studies on a series of D–π–A structured porphyrin sensitizers with different electron-donor groups
Abstract
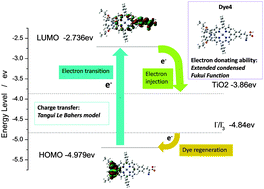
A series of D–π–A zinc porphyrin sensitizers Dye1–Dye6 bearing a substituted iminodibenzyl group at the porphyrin meso position, which is expected to have different electron-donating abilities, were designed. Theoretical studies were performed to examine the photovoltaic properties of these molecules in dye-sensitized solar cells (DSSCs). In particular, the important concepts, the Fukui function and the extended condensed Fukui function, are employed to describe the electron-donating abilities accurately at the quantitative level. Tangui Le Bahers model was adopted to analyze charge transfer (CT) during electron transition. A correlation between the electron donating abilities of the derived iminodibenzyl group and CT was built to evaluate the cell performance based on sensitizers Dye1–Dye6. The theoretical studies showed that porphyrins Dye1–Dye3 bearing an extremely strong electron-donating group (EDG) would fail in the generation of photocurrent in the closed circuit when applied in DSSCs due to the higher level of the HOMO energy than the redox potential of the redox couple (I−/I3−). The one with a weaker EDG (Dye4) is expected to show better photovoltaic performance than porphyrin IDB with an unsubstituted iminodibenzyl group. This study demonstrates a reliable method involving the employment of the Fukui function, the extended condensed Fukui function and the Tangui Le Bahers model for the evaluation of newly designed D–π–A type porphyrin sensitizers for use in DSSCs, and as guidance for future molecular design.

 Please wait while we load your content...
Please wait while we load your content...