Improving the electrochemical properties of LiNi0.5Co0.2Mn0.3O2 at 4.6 V cutoff potential by surface coating with Li2TiO3 for lithium-ion batteries
Abstract
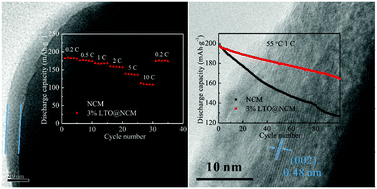
The Li2TiO3-coated LiNi0.5Co0.2Mn0.3O2 (LTO@NCM) cathode materials are synthesized via an in situ co-precipitation method followed by the lithiation process and thermal annealing. The Li2TiO3 coating layer is designed to strongly adhere to the core-material with 3D diffusion pathways for Li+ ions. Electrochemical tests suggest that compared with pristine NCM, Li2TiO3 serves as both a Li ion conductive layer and a protective coating layer against the attack of HF in the electrolyte, and remarkably improves the cycling performance at higher charged state and rate capability of the LTO@NCM composite material. What is more, phase transformation of NCM and dissolution of metal ions at high-temperatures at 4.6 V cutoff potential are effectively suppressed after LTO-coating. Our study demonstrates that LTO-coating on the surface of NCM is a viable method to improve the electrochemical performance of NCM, especially at high rates and under high-voltage charged conditions.

 Please wait while we load your content...
Please wait while we load your content...