Acidity of two-dimensional zeolites†
Abstract
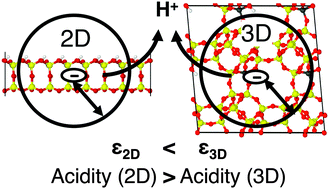
Hybrid quantum mechanics:molecular mechanics (QM/MM) calculations of absolute deprotonation energies are performed with periodic boundary conditions for Brønsted sites of aluminosilicate bilayers with various Al/Si ratios (two-dimensional zeolite). The supercell method is applied and density functional theory is used. Much lower values are obtained (1042, 1069 and 1091 kJ mol−1 for Al/Si = 1/63, 1/7 and 1/3, respectively) than those for bulk zeolites (1233 kJ mol−1 for H-chabazite with Al/Si = 1/11). We ascribe the much lower deprotonation energy to the smaller effective dielectric constant (1.6–1.9) of an ultra-thin dielectric in a vacuum compared to that of the corresponding bulk systems (3.0 for H-chabazite), which leads to a better stabilization of the charge created upon deprotonation.

 Please wait while we load your content...
Please wait while we load your content...