The role of outer surface/inner bulk Brønsted acidic sites in the adsorption of a large basic molecule (simazine) on H-Y zeolite
Abstract
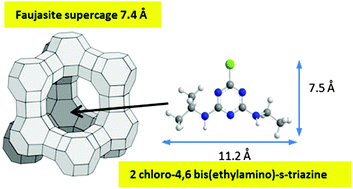
The simple means adopted for investigating H-Y zeolite acidity in water is the pH-dependence of the amount of a basic molecule adsorbed under isochoric conditions, a technique capable of yielding, under equilibrium conditions, an estimate of the pKa value of the involved acidic centres: the behaviour with temperature of adsorbed amounts yields instead some information on thermodynamics. Simazine (Sim, 2-chloro-4,6-bis(ethylamino)-s-triazine) was chosen as an adsorbate because its transverse dimension (7.5 Å) is close to the opening of the supercage in the faujasite structure of H-Y (7.4 Å). In short term measurements, Sim adsorption at 25 °C occurs only at the outer surface of H-Y particles. Two types of mildly acidic centres are present (with pKaca. 7 and ca. 8, respectively) and no strong one is observed. Previous adsorption of ammonia from the gas phase discriminates between the two. The former survives, and shows features common with the silanols of amorphous silica. The latter is suppressed: because of this and other features distinguishing this site from silanol species (e.g. the formation of dimeric Sim2H+ species, favoured by coverage and unfavoured by temperatures of adsorption higher than ambient temperature) a candidate is an Al based site. We propose a Lewis centre coordinating a water molecule, exhibiting acidic properties. This acidic water molecule can be replaced by the stronger base ammonia, also depleting inner strong Brønsted sites. A mechanism for the generation of the two sites from surface Brønsted species is proposed. Long term adsorption measurements at 25 °C already show the onset of the interaction with inner strongly acidic Brønsted sites: because of its size, activation is required for Sim to pass the supercage openings and reach inner acidic sites. When adsorption is run at 40–50 °C, uptake is much larger and increases with temperature. Isochoric measurements suggest a pKa value of ca. 3 compatible with its marked acidic nature, although attainment of equilibrium conditions is questionable. Measurements at 60 °C (both isochors and DRIFT) show the onset of changes at the outer surface brought about by the presence of hot water. Control experiments run with USY (Ultra Stabilized zeolite Y), featuring wormholes and cavities rendering accessible internal sites, show the extensive involvement of internal Brønsted sites already at 25 °C.

 Please wait while we load your content...
Please wait while we load your content...