A comparative first principles study on trivalent ion incorporated SSZ-13 zeolites†
Abstract
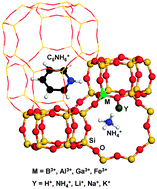
The dispersion-corrected density functional theory has been used to study the trivalent ions B, Al, Ga, and Fe incorporated SSZ-13-type zeolites. The associated structure and Brønsted/Lewis acidity change caused by the incorporation ions were comparatively studied. It was found that the smaller the radius differences of the incorporation ions are, the smaller the changes in the structure will be and the less acidity will be enhanced for the Brønsted sites. The trivalent Al is found to be the most favorable trivalent incorporation ion and Na is found to be the most favorable charge balanced ion for the synthesis of SSZ-13-type zeolites due to size comparability, which are in line with the experimental observation. The substitution energies which show the relative synthesis difficulty level were also applied for B, Al, Ga, and Fe incorporated zeolites and found that the difficulty decreases with order of Fe > B > Ga ≫ Al, also in good agreement with the experimental observations. Adsorption studies for the NH3 and pyridine molecules indicate that adsorption on the Brønsted acid sites is more stable than on the Lewis acid sites. The Brønsted acidity was found to follow the order of HAl-SSZ-13 > HGa-SSZ-13 ≈ HFe-SSZ-13 > HB-SSZ-13 where the Lewis acidity was found to follow the order of HGa-SSZ-13 ≈ HFe-SSZ-13 > HAl-SSZ-13 > HB-SSZ-13. Our results provide new insights for the synthesis of the SSZ-13-type zeolites and fundamental information for the zeolitic catalyst designation to enhance the catalytic performance.

 Please wait while we load your content...
Please wait while we load your content...