Investigation of Fickian diffusion in the ternary mixtures of water–ethanol–triethylene glycol and its binary pairs
Abstract
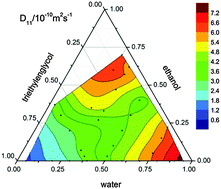
We present a comprehensive experimental study of isothermal Fickian diffusion in the ternary and binary liquid mixtures of water, ethanol, and triethylene glycol over the entire ternary composition space. 21 ternary mixtures inside the composition triangle have been investigated by means of the Taylor dispersion technique and 30 binary mixtures by Taylor dispersion and/or optical beam deflection in a Soret cell. The scalar binary diffusion coefficient has been determined along all three binary boundaries of the composition space and compared with estimations based on the Stokes–Einstein relation using stick or slip boundary conditions. The four elements of the ternary diffusion matrix and the diffusion eigenvalues were determined over a large portion of the composition triangle. The pseudo-binary diffusion coefficients obtained in Taylor dispersion experiments with either one of the two independent concentrations kept constant are comparable to the two diffusion eigenvalues. One of the two off-diagonal elements of the diffusion matrix is of the same order as the diagonal ones and, hence, not negligible, whereas the other one is approximately one order of magnitude smaller. Where available, our results compare well with literature data. The investigated compositions also comprise the five compositions that are scheduled for microgravity experiments in the ESA DCMIX3 project.

 Please wait while we load your content...
Please wait while we load your content...