Lithium-doping inverts the nanoscale electric field at the grain boundaries in Cu2ZnSn(S,Se)4 and increases photovoltaic efficiency†
Abstract
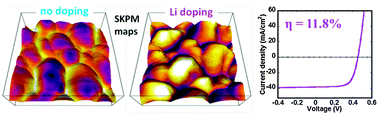
Passive grain boundaries (GBs) are essential for polycrystalline solar cells to reach high efficiency. However, the GBs in Cu2ZnSn(S,Se)4 have less favorable defect chemistry compared to CuInGaSe2. Here, using scanning probe microscopy we show that lithium doping of Cu2ZnSn(S,Se)4 changes the polarity of the electric field at the GB such that minority carrier electrons are repelled from the GB. Solar cells with lithium-doping show improved performance and yield a new efficiency record of 11.8% for hydrazine-free solution-processed Cu2ZnSn(S,Se)4. We propose that lithium competes for copper vacancies (forming benign isoelectronic LiCu defects) decreasing the concentration of ZnCu donors and competes for zinc vacancies (forming a LiZn acceptor that is likely shallower than CuZn). Both phenomena may explain the order of magnitude increase in conductivity. Further, the effects of lithium doping reported here establish that extrinsic species are able to alter the nanoscale electric fields near the GBs in Cu2ZnSn(S,Se)4. This will be essential for this low-cost Earth abundant element semiconductor to achieve efficiencies that compete with CuInGaSe2 and CdTe.

 Please wait while we load your content...
Please wait while we load your content...