Effects of the amino acid sequence on thermal conduction through β-sheet crystals of natural silk protein†
Abstract
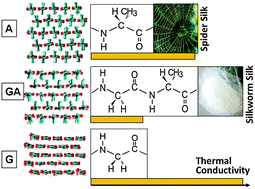
Recent experiments have discovered very different thermal conductivities between the spider silk and the silkworm silk. Decoding the molecular mechanisms underpinning the distinct thermal properties may guide the rational design of synthetic silk materials and other biomaterials for multifunctionality and tunable properties. However, such an understanding is lacking, mainly due to the complex structure and phonon physics associated with the silk materials. Here, using non-equilibrium molecular dynamics, we demonstrate that the amino acid sequence plays a key role in the thermal conduction process through β-sheets, essential building blocks of natural silks and a variety of other biomaterials. Three representative β-sheet types, i.e. poly-A, poly-(GA), and poly-G, are shown to have distinct structural features and phonon dynamics leading to different thermal conductivities. A fundamental understanding of the sequence effects may stimulate the design and engineering of polymers and biopolymers for desired thermal properties.

 Please wait while we load your content...
Please wait while we load your content...