Particularly strong C–H⋯π interactions between benzene and all-cis 1,2,3,4,5,6-hexafluorocyclohexane†
Abstract
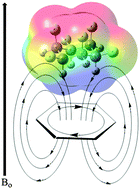
We present the first high-level ab initio benchmark study of the interaction energy between fluorocyclohexanes and benzene. These compounds form CH⋯π interactions with aromatic solvents which causes notable shielding of the axial cyclohexane protons. For the recently synthesised all-cis 1,2,3,4,5,6-hexafluorocyclohexane the interaction energy with benzene amounts to −7.9 kcal mol−1 and −6.4 kcal mol−1 at the MP2 and SCS-MP2 levels, respectively (extrapolated to the complete basis set limit), which according to dispersion-corrected density functional calculations, is largely due to dispersion.

 Please wait while we load your content...
Please wait while we load your content...