Oxygen reduction and oxygen evolution in DMSO based electrolytes: the role of the electrocatalyst†
Abstract
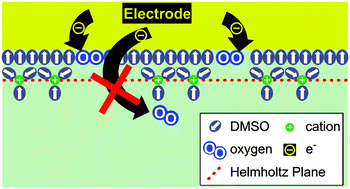
In the present paper the role of the electrode material in oxygen reduction in DMSO based electrolytes is elucidated using DEMS. We have found, employing platinum, gold, ruthenium rhodium, selenium decorated rhodium and boron doped diamond (BDD) as electrode materials, that the actual mechanism of oxygen reduction largely depends on the electrode material. At platinum, rhodium and selenium decorated rhodium the final reduction product, peroxide, is formed electrochemically. At gold and at low overpotentials oxygen is reduced to superoxide and peroxide is only formed by disproportionation of the latter. No oxygen reduction takes place at the diamond surface of the BDD-electrode, hence, showing unambiguously that oxygen reduction is an inner sphere reaction. Also, the rate of oxygen evolution varies with the electrode material, although the onset potential of oxygen evolution is not influenced. The amount of peroxide formed is limited to 1–2 monolayers. Contrary to intuition oxygen reduction and oxygen evolution from peroxide, therefore, are heterogeneous, electrocatalytic reactions. The finding of such an electrocatalytic effect is of great importance for the development and optimization of lithium–air batteries. Aside from the electrode material there are also effects of water as well as of the cation used in the electrolyte. This suggests an influence of the double layer at the interface between the electrode and the electrolyte on oxygen reduction in addition to the well-known higher stability of Na2O2 and K2O2. Electrospray ionization (ESI) results show that any effect of water in the Li+ containing electrolyte is not due to an altered solvation of the cation.

 Please wait while we load your content...
Please wait while we load your content...