Weak intramolecular and intermolecular hydrogen bonding of benzyl alcohol, 2-phenylethanol and 2-phenylethylamine in the adsorption on graphitized thermal carbon black†
Abstract
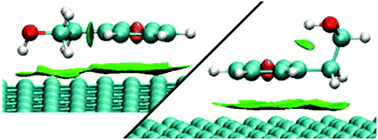
The paper discusses the role of weak intra- and intermolecular hydrogen bonds in the adsorption of benzyl alcohol, 2-phenylethanol and 2-phenylethylamine on graphitized thermal carbon black (GTCB). Using the B3LYP/aug-cc-pVDZ, B3LYP/aug-cc-pVTZ methods and the molecular-statistical theory of adsorption we found the structural and energetic parameters of the conformers to be stable in the gas phase and in the adsorbed state. The contribution of weak OH⋯π, NH⋯π, CH⋯O hydrogen bonds to the stabilization of the conformers was defined by the method of non-covalent interactions (NCI). Based on the difference in the experimental and calculated values of the Henry constant Δ ln K1 < 0.25 (K1, cm3 m−2) a high predictive power of molecular-statistical calculation of the thermodynamic characteristics of adsorption (TCA) has been shown. To obtain a high predictive power of molecular-statistical calculation it was necessary to take due account of the structural features of flexible molecules in the adsorbed state. A significant impact of the weak OH⋯πGTCB intermolecular hydrogen bond of benzyl alcohol on the TCA values has been established.

 Please wait while we load your content...
Please wait while we load your content...