Unwilling U–U bonding in U2@C80: cage-driven metal–metal bonds in di-uranium fullerenes†
Abstract
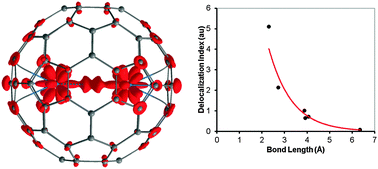
Endohedral actinide fullerenes are rare and a little is known about their molecular properties. Here we characterize the U2@C80 system, which was recently detected experimentally by means of mass spectrometry (Akiyama et al., JACS, 2001, 123, 181). Theoretical calculations predict a stable endohedral system, 7U2@C80, derived from the C80:7 IPR fullerene cage, with six unpaired electrons. Bonding analysis reveals a double ferromagnetic (one-electron-two-center) U–U bond at an rU–U distance of 3.9 Å. This bonding is realized mainly via U(5f) orbitals. The U–U interaction inside the cage is estimated to be about −18 kcal mol−1. U–U bonding is further studied along the U2@Cn (n = 60, 70, 80, 84, 90) series and the U–U bonds are also identified in U2@C70 and U2@C84 systems at rU–U ∼ 4 Å. It is found that the character of U–U bonding depends on the U–U distance, which is dictated by the cage type. A concept of unwilling metal–metal bonding is suggested: uranium atoms are strongly bound to the cage and carry a positive charge. Pushing the U(5f) electron density into the U–U bonding region reduces electrostatic repulsion between enclosed atoms, thus forcing U–U bonds.


 Please wait while we load your content...
Please wait while we load your content...