Unraveling the effect of La A-site substitution on oxygen ion diffusion and oxygen catalysis in perovskite BaFeO3 by data-mining molecular dynamics and density functional theory†
Abstract
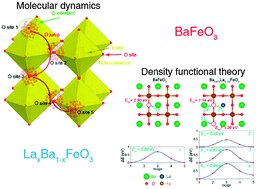
BaFeO3 (BFO) is a promising parent material for high-temperature oxygen catalysis. The effects of La substitution on the oxygen ion diffusion and oxygen catalysis in A-site La-substituted BFO are studied by combining data-driven molecular dynamics (MD) simulations and density functional theory (DFT) calculations. The data-driven MD simulations are capable of providing atomic level information regarding oxygen jumps at different sites, bridging the resolution gap of analysis between MD and DFT. The simulations identify several effects due to the introduction of La. First, according to simple electroneutrality considerations and DFT calculations, La tends to decrease the concentration of oxygen vacancies in BFO. Second, La substitution lowers the activation energy of local oxygen migration, providing faster paths for oxygen diffusion. The MD analysis predicts a higher hopping rate through La-containing bottlenecks as well as easier oxygen jumps from the La-rich cages and lower dwell times of oxygen in those cages. DFT calculations confirm a lower migration energy through La-containing bottlenecks. Third, the electrocatalytic activity of the material decreases with La, as indicated by a lower O p-band center and higher oxygen vacancy formation energies.

 Please wait while we load your content...
Please wait while we load your content...