Theoretical study of the reactions of Criegee intermediates with ozone, alkylhydroperoxides, and carbon monoxide†
Abstract
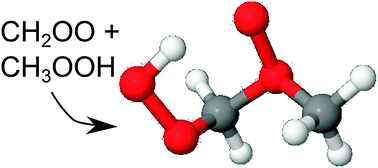
The reaction of Criegee intermediates (CI) with ozone, O3, has been re-examined with higher levels of theory, following earlier reports that O3 could be a relevant sink of CI. The updated rate coefficients indicate that the reaction is somewhat slower than originally anticipated, and is not expected to play a role in the troposphere. In experimental (laboratory) conditions, the CI + O3 reaction can be important. The reaction of CI with ROOH intermediates is found to proceed through a pre-reactive complex, and the insertion process allows for the formation of oligomers in agreement with recent experimental observations. The CI + ROOH reaction also allows for the formation of ether oxides, which don't react with H2O but can oxidize SO2. Under tropospheric conditions, the ether oxides are expected to re-dissociate to the CI + ROOH complex, and ultimately follow the insertion reaction forming a longer-chain hydroperoxide. The CI + ROOH reaction is not expected to play a significant role in the atmosphere. The reaction of CI with CO molecules was studied at very high levels of theory, but no energetically viable route was found, leading to very low rate coefficients. These results are compared against an extensive literature overview of experimental data.

 Please wait while we load your content...
Please wait while we load your content...