Precipitation and surface adsorption of metal complexes during electropolishing. Theory and characterization with X-ray nanotomography and surface tension isotherms†
Abstract
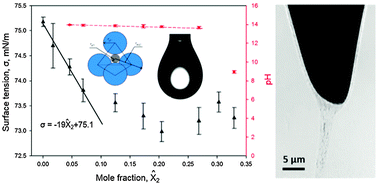
Electropolishing of metals often leads to supersaturation conditions resulting in precipitation of complex compounds. The solubility diagrams and Gibbs adsorption isotherms of the electropolishing products are thus very important to understand the thermodynamic mechanism of precipitation of reaction products. Electropolishing of tungsten wires in aqueous solutions of potassium hydroxide is used as an example illustrating the different thermodynamic scenarios of electropolishing. Electropolishing products are able to form highly viscous films immiscible with the surrounding electrolyte or porous shells adhered to the wire surface. Using X-ray nanotomography, we discovered a gel-like phase formed at the tungsten surface during electropolishing. The results of these studies suggest that the electropolishing products can form a rich library of compounds. The surface tension of the electrolyte depends on the metal oxide ions and alkali-metal complexes.

 Please wait while we load your content...
Please wait while we load your content...