Controlling charge injection properties in polymer field-effect transistors by incorporation of solution processed molybdenum trioxide†
Abstract
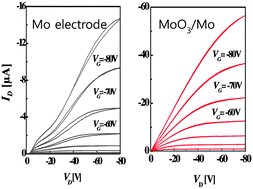
A simply and facilely synthesized MoO3 solution was developed to fabricate charge injection layers for improving the charge-injection properties in p-type organic field-effect transistors (OFETs). By dissolving MoO3 powder in ammonium (NH3) solvent under an air atmosphere, an intermediate ammonium molybdate ((NH4)2MoO4) precursor is made stable, transparent and spin-coated to form the MoO3 interfacial layers, the thickness and morphology of which can be well-controlled. When the MoO3 layer was applied to OFETs with a cost-effective molybdenum (Mo) electrode, the field-effect mobility (μFET) was significantly improved to 0.17 or 1.85 cm2 V−1s−1 for polymer semiconductors, regioregular poly(3-hexylthiophene) (P3HT) or 3,6-bis-(5bromo-thiophen-2-yl)-N,N′-bis(2-octyl-1-dodecyl)-1,4-dioxo-pyrrolo[3,4-c]pyrrole (DPPT-TT), respectively. Device analysis indicates that the MoO3-deposited Mo contact exhibits a contact resistance RC of 1.2 MΩ cm comparable to that in a device with the noble Au electrode. Kelvin-probe measurements show that the work function of the Mo electrode did not exhibit a dependence on the thickness of MoO3 film. Instead, ultraviolet photoemission spectroscopy results show that a doping effect is probably induced by casting the MoO3 layer on the P3HT semiconductor, which leads to the improved hole injection.

 Please wait while we load your content...
Please wait while we load your content...