Anomalous system-size dependence of electrolytic cells with an electrified oil–water interface
Abstract
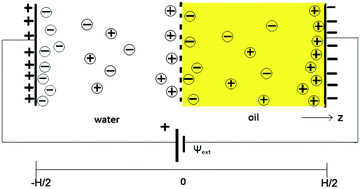
Manipulation of the charge of the dielectric interface between two bulk liquids not only enables the adjustment of the interfacial tension but also controls the storage capacity of ions in the ionic double layers adjacent to each side of the interface. However, adjusting this interfacial charge by static external electric fields is difficult since the external electric fields are readily screened by ionic double layers that form in the vicinity of the external electrodes. This leaves the liquid–liquid interface, which is at a macroscopic distance from the electrodes, unaffected. In this study we show theoretically, in agreement with recent experiments, that control over this surface charge at the liquid–liquid interface is nonetheless possible for macroscopically large but finite closed systems in equilibrium, even when the distance between the electrode and interface is orders of magnitude larger than the Debye screening lengths of the two liquids. We identify a crossover system-size below which the interface and the electrodes are effectively coupled. Our calculations of the interfacial tension for various electrode potentials are in good agreement with recent experimental data.

 Please wait while we load your content...
Please wait while we load your content...