Slow dynamics of water confined in Newton black films†
Abstract
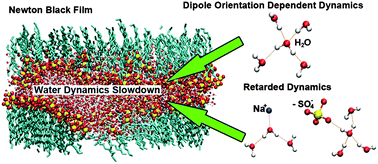
Slowdown of translational and reorientational dynamics of water confined in Newton black films (NBFs) is revealed by molecular dynamics simulations. As a film becomes thinner, both translational and reorientational dynamics become slower. The polarization of water molecules in the macroscopic electrostatic field across the NBF and the coordination of Na+ ions and surfactant anionic groups around water molecules concertedly lead to slowdown of water dynamics. The polarization effect is obvious for water not coordinated by Na+ ions, which exhibits reorientational dynamics depending on initial dipole orientations. Na+ ions and surfactant anionic groups retard dynamics of surrounding water by decreasing the hydrogen bond exchange probability and increasing the viscosity of water. The dependences of translational and reorientational dynamics on coordination environments of water are similar. Dynamics of water in positions close to the interfaces of NBFs are mainly retarded by Na+ ions and surfactant anionic groups, while the macroscopic polarization effect plays the main role in influencing water dynamics in positions far from the interfaces. This study sheds light on the improvement of knowledge about the water dynamics slowdown mechanism in similar environments like reverse micelles and lamellar structures.

 Please wait while we load your content...
Please wait while we load your content...