A photoionization mass spectroscopic study on the formation of phosphanes in low temperature phosphine ices†
Abstract
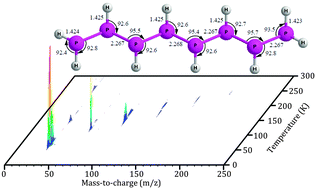
Isovalency rationalizes fundamental chemical properties of elements in the same group, but often fails to account for differences in the molecular structure due to the distinct atomic sizes and electron-pair repulsion of the isovalent atoms. With respect to main group V, saturated hydrides of nitrogen are limited to ammonia (NH3) and hydrazine (N2H4) along with ionic and/or metal-bound triazene (N3H5) and potentially tetrazene (N4H6). Here, we present a novel approach for synthesizing and detecting phosphanes formed via non-classical synthesis exploiting irradiation of phosphine ices with energetic electrons, subliming the newly formed phosphanes via fractionated sublimation, and detecting these species via reflectron time-of-flight mass spectrometry (ReTOF) coupled with vacuum ultraviolet (VUV) single photon ionization. This approach is able to synthesize, to separate, and to detect phosphanes as large as octaphosphane (P8H10), which far out-performs the traditional analytical tools of infrared spectroscopy and residual gas analysis via mass spectrometry coupled with electron impact ionization that could barely detect triphosphane (P3H5) thus providing an unconventional tool to prepare complex inorganic compounds such as a homologues series of phosphanes, which are difficult to synthesize via classical synthetic methods.

 Please wait while we load your content...
Please wait while we load your content...