Mechanochemistry of lithium nitride under hydrogen gas†
Abstract
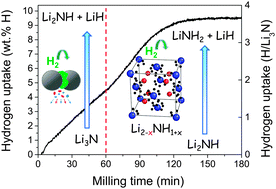
Hydrogen uptake during the mechanochemistry of lithium nitride under 9 MPa hydrogen pressure has been analyzed by means of in situ solid–gas absorption and ex situ X-ray diffraction (XRD) measurements. In situ hydrogenation curves show two H-sorption steps leading to an overall hydrogen uptake of 9.8 wt% H after 3 hours of milling. The milled end-products consist of nanocrystalline (∼10 nm) LiNH2 and LiH phases. The first reaction step comprises the transformation of the polymorph α-Li3N (S.G. P6/mmm) into the β-Li3N (S.G. P63/mmc) metastable phase and the reaction of the latter with hydrogen to form lithium imide: β-Li3N + H2 → Li2NH + LiH. Reaction kinetics of the first step is zero-order. Its rate-limiting control is assigned to the collision frequency between milling balls and Li3N powder. In the second absorption step, lithium imide converts to lithium amide following the reaction scheme Li2NH + H2 → LiNH2 + LiH. Reaction kinetics is here limited by one-dimensional nucleation and the growth mechanism, which, in light of structural data, is assigned to the occurrence of lithium vacancies in the imide compound. This study provides new insights into the reaction paths and chemical kinetics of light hydrogen storage materials during their mechanochemical synthesis.

 Please wait while we load your content...
Please wait while we load your content...