Two phosphonium ionic liquids with high Li+ transport number†
Abstract
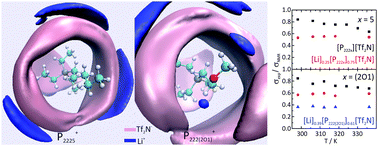
This work presents the physicochemical characterization of two ionic liquids (ILs) with small phosphonium cations, triethylpenthylphosphonium bis(trifluoromethanesulfonyl)imide ([P2225][Tf2N]) and (2-methoxyethyl)trimethylphosphonium bis(trifluoromethanesulfonyl)imide ([P222(201)][Tf2N]), and their mixtures with Li+. Properties such as the electrochemical window, density, viscosity and ionic conductivity are presented. The diffusion coefficient was obtained using two different techniques, PGSE-NMR and Li electrodeposition with microelectrodes. In addition, the Li+ transport number was calculated using the PGSE-NMR technique and an electrochemical approach. The use of these three techniques showed that the PGSE-NMR technique underestimates the diffusion coefficient for charged species. The Li+ transport number was found to be as high as 0.54. Raman spectroscopy and molecular dynamics simulations were used to evaluate the short-range structure of the liquids. These experiments suggested that the interaction between the Li+ and the Tf2N− anion is similar to that seen with other ILs containing the same anion. However, the MD simulations also showed that the Li+ ions interact differently with the cation containing an alkyl ether chain. The results found in this work suggest that these Li+ mixtures have promising potential to be applied as electrolytes in batteries.

 Please wait while we load your content...
Please wait while we load your content...