Evidence for the existence of Li2S2 clusters in lithium–sulfur batteries: ab initio Raman spectroscopy simulation
Abstract
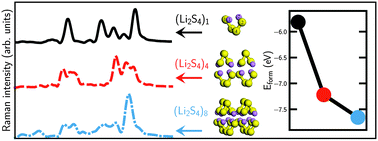
Using density functional theory calculations and ab initio molecular dynamics simulations we have studied the structures and the Raman spectra of Li2S4 clusters, which are believed to be the last polysulfide intermediates before the formation of Li2S2/Li2S during the discharge process in Li–S batteries. Raman spectra have been obtained using a new technique to estimate polarizabilities using Wannier functions. We have observed clear evidence of Li2S4 → Li2S2 transition by studying systematic changes in the simulated Raman spectra of (Li2S4)n, n = 1, 4, and 8 towards that of (Li2S2)8. Furthermore, we have shown that the dominant Raman peak of the Li2S2 cluster at ∼440 cm−1 arises from sulfur–sulfur stretching mode. This peak has been experimentally observed in the discharged state of Li–S batteries and has also been attributed to the formation of Li2S2. We have also demonstrated that the transition is mainly due to the strong electrostatic interactions between Li2S4 monomers, which results in energy lowering by arranging the local Li+δ–S−δ dipole moments in an anti-parallel fashion.

 Please wait while we load your content...
Please wait while we load your content...