The rotational barrier of ethane and some of its hexasubstituted derivatives in terms of the forces acting on the electron distribution†
Abstract
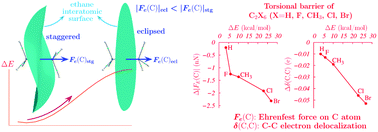
A novel and alternative explanation of the rotational barrier of ethane and several hexasubstituted derivatives, CX3CX3 (X = H, F, CH3, Cl, Br), is suggested based on the evaluation of the properties of the electron distribution. The forces exerted on the electron density of the topological atoms making up a molecule, the Ehrenfest forces, are analyzed and, with the help of the virial theorem, they are used to explain the experimental rotational barriers. According to this approach, the barrier is mainly a consequence of the decrease of the always attractive Ehrenfest forces (EFs) linking the two C atoms. In addition, the behavior of the EFs is related to a decrease of stability of the central C atoms, which is not compensated by the stabilization of the substituents. Also, during rotation from the staggered to the equilibrium conformation, the electron density at the C–C bond critical point and the electron delocalization between C atoms decrease and are accompanied by an increase of electron delocalization between the substituents. According to the analysis of the EF field lines and to the behavior of the integrated forces, the rotational barrier cannot be explained as a result of any repulsive forces acting on the electron density, although a possible interpretation of the quantum force that balances the EF in stationary states as a measure of traditional Pauli repulsions is also examined.

 Please wait while we load your content...
Please wait while we load your content...