Exploring PtSO4 and PdSO4 phases: an evolutionary algorithm based investigation†
Abstract
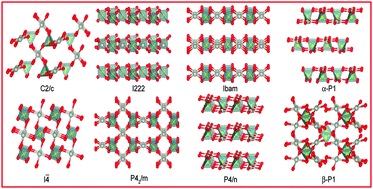
Metal sulfate formation is one of the major challenges to the emission aftertreatment catalysts. Unlike the incredibly sulfation prone nature of Pd to form PdSO4, no experimental evidence exists for PtSO4 formation. Given the mystery of nonexistence of PtSO4, we explore PtSO4 using a combined approach of an evolutionary algorithm based search technique and quantum mechanical computations. Experimentally known PdSO4 is considered for the comparison and validation of our results. We predict many possible low-energy phases of PtSO4 and PdSO4 at 0 K, which are further investigated in a wide range of temperature–pressure conditions. An entirely new low-energy (tetragonal P42/m) structure of PtSO4 and PdSO4 is predicted, which appears to be the most stable phase of PtSO4 and a competing phase of the experimentally known monoclinic C2/c phase of PdSO4. Phase stability at a finite temperature is further examined and verified by Gibbs free energy calculations of sulfates towards their possible decomposition products. Finally, temperature–pressure phase diagrams are computationally established for both PtSO4 and PdSO4.

 Please wait while we load your content...
Please wait while we load your content...