The synergistic effect between effective mass and built-in electric field for the transfer of carriers in nonlinear optical materials
Abstract
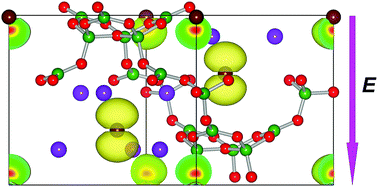
Recent experiments have demonstrated that the typical nonlinear optical material K3B6O10Br can be an excellent photocatalyst under ultraviolet (UV) light irradiation. To understand the origin of the photocatalytic activity and further improve its photocatalytic efficiency to develop alternative photocatalysts, the built-in electric field and the electron effective mass and their synergistic effect on transfer and the separation of carriers in K3B6O10X (X = Br, Cl) were investigated by means of first-principles calculations. Our results show that the built-in electric field and the smallest effective mass of holes in K3B6O10Br are both along the [001] direction. In contrast, the effective masses of electrons are isotropic because of the spherically symmetric s orbitals at the conduction band minimum (CBM). Therefore, the electric field can promote efficient transfer and separation of the photogenerated carriers along the [001] direction. As a consequence, the synergistic effect of built-in electric field and the isotropy of the electron effective mass results in the {001} surface, to which most of the carriers will accumulate, showing the highest photocatalytic activity. Similar results can also be obtained for a K3B6O10Cl crystal considering the analogous structure with that of K3B6O10Br. The present study may provide theoretical insight to develop the photocatalytic performance of nonlinear optical materials.

 Please wait while we load your content...
Please wait while we load your content...