Harvesting light energy by iridium(iii) complexes on a clay surface†
Abstract
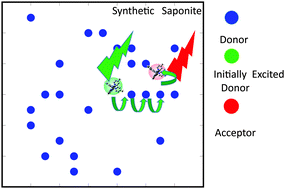
Energy transfer was investigated between two types of iridium(III) complexes, [Ir(dfppy)2(Cn-bpy)]+ (dfppyH = 2-(2′,4′-difluorophenyl)pyridine; Cn-bpy = 4,4′-dialkyl-2,2′-bipyridine; dialkyl = dimethyl (C1), didodecyl (C12), and dinonyldecyl (C19)) and [Ir(piq)2(Cn-bpy)]+ (piqH = 1-phenylisoquinoline) as a donor and an acceptor, respectively. The complexes were co-adsorbed by colloidally dispersed synthetic saponite. The efficiency of energy transfer (ηET) was obtained from emission spectra at various donor-to-acceptor ratios (D/A) on the basis of the Förster-type energy transfer mechanism. For C1-bpy, ηET was as high as 0.5 with a D/A of ca. 20. The results implied that the photon energy captured by several donor molecules was collected by a single acceptor molecule (i.e. the harvesting of light energy). Enantioselectivity was observed, which indicates the participation of a contact pair of donor and acceptor molecules. For C12-bpy and C19-bpy, ηET was low and exhibited no enantioselectivity, because their long alkyl chains inhibited close contact between the donor and acceptor molecules.

 Please wait while we load your content...
Please wait while we load your content...