Hydrothermal stability investigation of micro- and mesoporous silica containing long-range ordered cobalt oxide clusters by XAS
Abstract
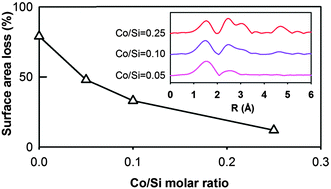
This work investigates the hydrothermal stability of cobalt doped silica materials with different Co/Si molar ratios (0, 0.05, 0.10, and 0.25). The resultant materials were characterized by N2 sorption and chemical structures by Raman and X-ray absorption spectroscopy before and after a harsh hydrothermal exposure (550 °C, 75 mol% vapour and 40 h). The cobalt silica materials showed a lower surface area loss from 48% to 12% with increasing Co/Si molar ratio from 0.05 to 0.25 and relatively maintaining their pore size distribution, while pure silica exhibited significant surface area reduction (80%) and pore size broadening. For low cobalt loading sample (Co/Si = 0.05), the cobalt was highly dispersed in the silica network in a tetrahedral coordination with oxygen and a small proportion of Co–Co interaction in the second shell. Long range order Co3O4 was observed when Co/Si molar ratio increased to 0.10 and 0.25. The hydrothermal exposure did not affect the local cobalt environments and no cobalt–silicon interaction was observed by X-ray absorption spectroscopy. The hydrothermal stability of the silica matrix was attributed to the physical barrier of cobalt oxide in opposing densification and silica mobility under harsh hydrothermal conditions.


 Please wait while we load your content...
Please wait while we load your content...