Diamond monohydride: the most stable three-dimensional hydrocarbon†
Abstract
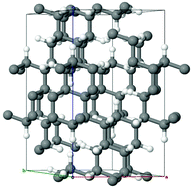
Most of the hydrocarbons are either molecular structures or linear polymeric chains. Discovery of graphene and manufacturing of its monohydride – graphane have incited interest in the search for three-dimensional hydrocarbon polymers. However, up to now all hypothetical hydrocarbon lattices significantly have lost in terms of energy to stacked graphane sheets and solid benzene. We propose a completely covalently bonded solid carbon monohydride, whose density significantly exceeds that of one of its isomers (graphane, cubane, and solid benzene). Ab initio calculations demonstrate that the cohesion energy of this structure at least is not worse than the energy of graphane and benzene. In some aspect, the crystal structure of the hydrocarbon presented can be regarded as a sublattice of diamond, but with the symmetry of the P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group (lattice parameters: a ≈ 6.925 Å and c ≈ 12.830 Å) and Z = 42 formula units per unit cell. This structure may have interesting applications.
space group (lattice parameters: a ≈ 6.925 Å and c ≈ 12.830 Å) and Z = 42 formula units per unit cell. This structure may have interesting applications.

 Please wait while we load your content...
Please wait while we load your content...