Theoretical survey of the ligand tunability of poly(azolyl)borates
Abstract
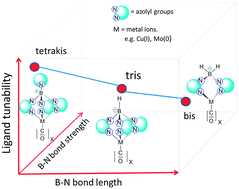
Using density functional calculations, we have systematically investigated a series of homoleptic poly(azolyl)borate ligands, which display unusual steadily declining bond strengths accompanied by bond contractions when the azolyl groups are sequentially substituted to the parent BH4−. As ligands, their effects on the coordinated metal ions (Cu(I) and Mo(0)) are quantitatively represented by two ligand tunability descriptors: the vibration frequency (νCO) of the CO groups complexed to the metal ions and the charge of the metal–(CO)x moiety, between which a good linear correlation exists. For the same number of azolyl substitutions, the boundary of ligand tunability is always marked by the pyrazolyl and tetrazolyl groups at the two ends, which feature the lowest and the highest nitrogen content in the azolyl ring, respectively. With the increase of the azolyl substitution number in the borate ligands, the νCO range expands, indicating a higher tunability of the ligands. The type of metal ion and the charge they carry play minor roles in influencing the ligand tunability.

 Please wait while we load your content...
Please wait while we load your content...