Activation of CO2 by ionic liquid EMIM–BF4 in the electrochemical system: a theoretical study†
Abstract
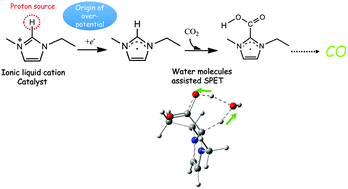
The electrochemical reduction of CO2 to CO by an ionic liquid EMIM–BF4 is one of the most promising CO2 reduction processes proposed so far with its high Faradaic efficiency and low overpotential. However, the details of the reaction mechanism are still unknown due to the absence of fundamental understandings. In this study, the most probable and stable geometries of EMIM–BF4 and CO2 were calculated by quantum chemistry in combination with exhaustive search. A possible reaction pathway from CO2 to CO catalyzed by EMIM–BF4, including the most plausible intermediates and the corresponding transition states, was proposed. The role of EMIM–BF4 is explained as forming a complex of [EMIM–COOH]− with CO2 followed by decomposing to CO.

 Please wait while we load your content...
Please wait while we load your content...